Speed Global's pharmaceutical cold chain customers are in central laboratories; drug research and development and sales; vaccines; biological reagents; stem cell immunotherapy; clinical medical and other institutions.
Products delivered include: blood samples; urine samples; DNA genes; muscle tissue sections; animals; plant specimens and clinical medications that require temperature control. Among them; in the fast medical cold chain transportation of stem cells and temperature control products; Tengyi Boshi is currently the most professional and one of the most accurate pharmaceutical cold chain logistics suppliers in China. We can assist you in the approval of relevant permits; filing and inspection by the Inspection and Quarantine Bureau; non-dangerous goods transportation certificate; agent declaration; temperature control product packaging plan; full temperature control record.
Regular service:
(1) Cold chain transportation of domestic medicine and life science drugs:
Can provide transportation temperature range: 2-8℃; 0-10℃; 15-25℃; 18-30℃; 0-30℃; -20 to -80℃ (dry ice); liquid nitrogen (-196℃); normal temperature Wait.
A. Provide a variety of time-limited services: 8 hours; 12 hours; 24 hours; next day delivery; next day delivery, etc.;
B. Provide multiple modes of transportation: air transportation; special car transportation; high-speed rail transportation; hand-held transportation, etc.;
C. Central cities provide warehousing services; to meet the needs of customers for warehousing management services in multiple locations.
(2) International life science cold chain transportation:
Relying on Speed Global's global emergency transportation network; it can handle life science cold chain transportation from all over the world to China and China to all over the world; it can provide 2-8℃; 15-25℃; 0-30℃; -20 to -80 ℃ (dry ice); transportation temperature control solutions at room temperature; products related to animal and plant tissue sections; medical biological reagents; blood samples; stem cells; other time-sensitive and temperature-sensitive products.
We can assist you in the approval of relevant license documents; non-dangerous goods transportation certification; agent inspection and filing, customs declaration and inspection; provide temperature-controlled product packaging solutions; real-time temperature control records throughout the process.

Why choose SPEED GLOBAL's pharmaceutical cold chain transportation?
(1) Intelligent temperature control: through the most modern Internet of Things technology; provide real-time monitoring of temperature control equipment; safety early warning; data analysis; on-site printing and other services.
(2) Professional packaging: Professional and experienced engineers will design and customize your proprietary cold chain packaging overall solution according to your product characteristics, transportation conditions and distance; let you enjoy convenience and ease.
(3) Safety and environmental protection: the packaging materials are all food-grade recyclable HDPE; PU materials; guaranteed non-toxic; harmless; will not cause pollution to the environment and sent items.
(4) Professional customer service: The professional customer service team provides customers with real-time cargo tracking service; you do not need to call for inquiries; the customer service will keep you updated on the status of the goods in a timely manner.
(5) Crisis handling: Standardized crisis handling procedures allow alternative plans or emergency remedial measures when accidents occur; making crisis handling calmer.

Medical cold chain transportation management and transportation operating procedures
First, the purpose
Formulate medical cold chain transportation storage and transportation management operating procedures; to ensure the effectiveness and safety of cold chain drugs.
2. Basis
"Pharmaceutical Management Law"; 2012 new version of "Pharmaceutical Operation Quality Management Regulations".
3. Scope of application
It is used for the quality risk control of cold chain medicines in the process of purchase; storage; sales and transportation.
4. Responsible person
Receiver; Acceptor; Warehouse clerk; Shipper; Transporter, etc.

Five, the content of the regulations
1. Terminology
①Refrigerated medicines refer to medicines that require temperature requirements such as storage, transportation, and freezing.
②Cold place refers to the storage and transportation conditions whose temperature meets 2℃~10℃. Unless otherwise specified; biological products should be stored at 2~8℃ and protected from light; transported.
③Freezing refers to storage at a temperature of -2°C and below; transportation conditions.
④Cold chain refers to temperature-sensitive drugs such as refrigerated drugs
From the finished product warehouse of the manufacturer to the entire storage before use; the circulation process must be under a specified temperature environment; a special supply chain management system to ensure the quality of medicines. The temperature and humidity automatic monitoring system in the cold storage will automatically record the actual value of temperature and humidity at least once every 10 minutes; the data should be true; complete; accurate; valid; readable; each measuring point data is automatically transmitted through the network; the record is kept for at least 5 years.
⑤The temperature control system includes an active temperature control system and a passive temperature control system. Active temperature control system refers to facilities and equipment with electromechanical instrument components to control the temperature; it is adjusted by program operation; the storage of medicines is controlled; the transportation temperature is within the set range. Passive temperature control system refers to equipment that controls temperature through non-electromechanical methods; such as incubators.

2. Personnel training management
①Receipt of medical cold chain transportation; acceptance; storage; maintenance; packing; operators involved in each link of transportation; all should be trained by the relevant quality management department and logistics management department, and be familiar with laws and regulations; cold chain basics Knowledge; the temperature sensitivity characteristics of the refrigerated medicines handled; the characteristics of product distribution and other cold chain management content.
②The operation of refrigeration (heat preservation) cabinet; use; maintenance personnel must be trained in the temperature sensitivity characteristics of the product; applicable conditions of the cabinet; pre-cooling conditions of the cold storage agent.
③The use of computer systems involved in cold chain management; the use of temperature recorders, etc.; relevant personnel should be trained; only after qualified training can they start their jobs.
④Personnel engaged in the receipt of refrigerated medicines; acceptance inspection; storage; maintenance; delivery; transportation; training in the storage of refrigerated medicines; transportation; emergency handling of emergencies.
⑤ Training plans and training files for operators and management personnel should be established; the effectiveness and adequacy of training should be evaluated regularly.

3. Receipt of medical cold chain transportation; acceptance management
①The receiving, shipping and loading area of refrigerated medicines should be set up in a cool place; should not be placed in direct sunlight; near heat source equipment or other locations that may increase the temperature of the surrounding environment.
② Check the real-time temperature record during the transportation of the medicine when receiving the goods; and use the temperature detector to detect the temperature.
③When receiving refrigerated medicines, you should ask for the delivery order; make real-time temperature records; and sign for confirmation. There are multiple handover links; each handover link must sign a handover order.
④The time for the refrigerated medicine to be transferred from the receiving area to the waiting area that meets the temperature requirements; the cold place medicine should be within 30 minutes, and the frozen medicine should be within 15 minutes.
⑤ Acceptance should be carried out in a refrigerated environment; acceptance of qualified drugs should be quickly transferred to the storage environment specified in the instructions.
⑥For returned drugs, the recipient shall be deemed to have received the goods; operate strictly in accordance with the above requirements of this article; and make records; if necessary, send it to the inspection department for inspection.
⑦Receipt of refrigerated medicines; delivery and acceptance records should be kept for 1 year after the expiry date of refrigerated medicines for future reference; the records shall be kept for at least 5 years.
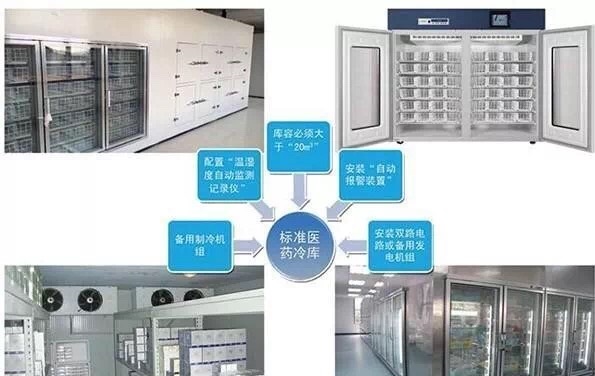
4. Medical cold chain transportation and storage; maintenance management
① The storage temperature of refrigerated drugs should meet the storage temperature requirements specified in the refrigerated drug instructions.
②When storing refrigerated medicines, the refrigerated medicines should be stored according to their varieties; the batch numbers should be classified and coded. There should be a certain distance for the drug stacking. The distance between the medicine and the wall and roof (room beam) is not less than 30 cm, the distance from the temperature control equipment in the warehouse is not less than 30 cm, and the distance from the ground is not less than 10 cm.
③Refrigerated medicines should be maintained and checked in the warehouse according to regulations and recorded. If abnormal quality is found; lock it first; isolate; suspend delivery; make a record; report to the quality management department for inspection in time; send it to the inspection department for inspection if necessary; and deal with it according to the inspection results.
④Maintenance records should be kept until 1 year after the expiration date of refrigerated drugs for future reference; the records should be kept for at least 5 years.
Previous: Cold chain drug transportation